Signed in as:
filler@godaddy.com
Signed in as:
filler@godaddy.com
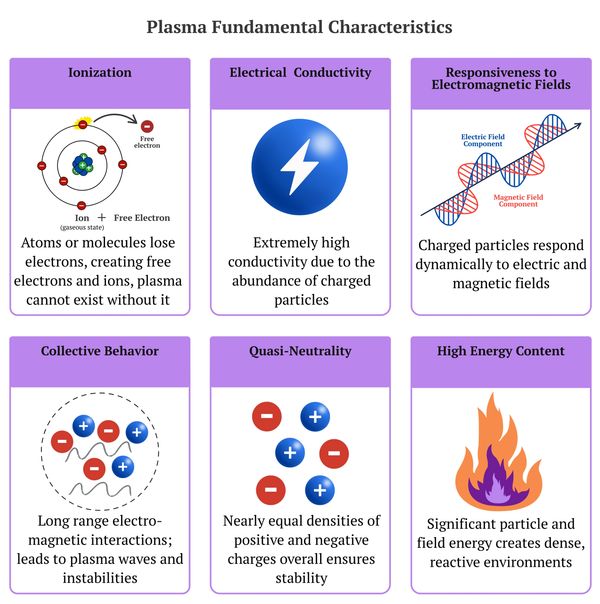
Plasma, often referred to as the fourth state of matter, is fundamentally distinct from the traditional states of matter (solid, liquid, and gas) due to its unique properties, which arise from its highly ionized nature and complex interactions with electromagnetic fields. These defining characteristics include a significant degree of ionization, high electrical conductivity, responsiveness to external electromagnetic fields, collective behavior driven by long-range Coulomb interactions, quasi-neutrality (a near balance of positive and negative charges on a macroscopic scale), and high-energy content. These traits enable plasma to exhibit behaviors and interactions that are vastly different from those of conventional matter, such as forming intricate structures and sustaining self-organized, dynamic processes.
Understanding how these characteristics arise and their impact on plasma behavior is crucial for advancing both scientific knowledge and practical applications. Plasma research has broad-reaching implications for various fields, including energy generation (e.g., nuclear fusion), industrial manufacturing (e.g., plasma etching and coatings), space exploration (e.g., astrophysical plasma dynamics and spacecraft propulsion), and medical technologies (e.g., plasma-based sterilization and therapies).
Natural and artificial plasmas often display notable differences in their properties and behaviors due to factors such as their environmental conditions, energy sources, and degrees of ionization. Artificial plasmas, in particular, exhibit a wide range of characteristics depending on their thermal regimes, which are broadly categorized into hot, warm, and cold plasmas. This diversity makes the study of natural and artificial plasmas crucial for understanding their shared principles while also addressing their unique applications and challenges.
The fundamental characteristics of plasma—including ionization, electrical conductivity, responsiveness to electromagnetic fields, collective behavior, quasi-neutrality, and high-energy content—distinguish it from conventional states of matter and provide a framework for understanding its unique behaviors and interactions. These characteristics occur from the ionized nature of plasma and the dynamic interplay of charged particles with electromagnetic forces. Their importance extends far beyond theoretical understanding, forming the foundation for innovations in various scientific and technological fields.
In natural plasmas, such as those found in stars, the interstellar medium, and planetary magnetospheres, these properties shape large-scale phenomena like energy transfer, wave propagation, and structure formation. On the other hand, artificial plasmas, which are engineered for specific conditions and purposes, allow researchers and engineers to explore and manipulate these fundamental characteristics in controlled environments. The ability to adjust parameters such as energy input, ionization levels, and electromagnetic interactions enables the harnessing of plasma for diverse applications, including fusion energy, material processing, and propulsion systems.
For example, ionization is crucial for understanding how plasma forms and behaves, while its enormous electrical conductivity underpins technologies like plasma-based lighting and fusion reactors. The responsiveness of plasma to electromagnetic fields is essential in stabilizing plasma in tokamak devices or guiding plasma flows in propulsion systems. Meanwhile, collective behavior and quasi-neutrality enable plasma to support waves and instabilities, which are key to understanding both cosmic plasmas and optimizing controlled fusion. Ultimately, the high-energy density of plasma drives cutting-edge technologies, such as plasma cutting, and supports ambitions for clean and efficient energy production.
Studying both natural and artificial plasmas sheds light on universal principles that connect the behavior of plasma in astrophysical settings with its engineered counterparts. These insights are crucial for addressing the distinct challenges presented by various plasma environments. Whether advancing humanity’s understanding of the universe or fostering high-impact innovations on Earth, plasma’s defining characteristics remain fundamental to its relevance and transformative potential.
Copyright © 2025 What is Cold Plasma? - All Rights Reserved.