Signed in as:
filler@godaddy.com
Signed in as:
filler@godaddy.com
Plasma, often referred to as the fourth state of matter, is characterized by its unique combination of charged particles, atoms, and neutral molecules that interact dynamically with one another. These fundamental properties give rise to plasma's derived characteristics, which are the secondary or emergent traits that result from the interactions of plasma's components. These unique features underpin plasma's wide-ranging applications in industrial, medical, and environmental fields. By examining plasma's derived traits—such as uniformity, stability, density, chemical composition, reactivity, energy transfer efficiency, selectivity in chemical reactions, material interactions, unique glow, and various states of molecular species—its unique behavior and how it supports advanced technologies are better understood.
The derived characteristics explored here—spanning uniformity, stability, reactivity, energy efficiency, and more—highlight the innovations enabled by cold plasma while elucidating how it differs fundamentally from its warm and hot counterparts. Cold plasma's ability to combine high electron energies with low bulk temperatures drives its impact on fields such as biomedicine, electronics manufacturing, environmental remediation, and beyond.
The derived characteristics of cold plasma, including its uniformity, stability, moderate density, reactive chemical composition, and precise energy transfer, distinguish it as a transformative tool for modern technologies. Unlike its hot and warm plasma counterparts, cold plasma's low thermal footprint, coupled with its high selectivity and efficiency, allows for innovative, energy-conscious applications in fields ranging from medicine to manufacturing. These derived traits enhance the technological potential of cold plasma while pushing the boundaries of material science, environmental sustainability, and precision medicine.
Plasma is distinguished by its ionized particles and interaction with electromagnetic fields. The uniformity of plasma—encompassing the consistency of key properties such as temperature, density, and composition—is critical to its performance and applications, especially in precision-driven industries. Artificial plasma systems are engine
Plasma is distinguished by its ionized particles and interaction with electromagnetic fields. The uniformity of plasma—encompassing the consistency of key properties such as temperature, density, and composition—is critical to its performance and applications, especially in precision-driven industries. Artificial plasma systems are engineered with a focus on achieving high uniformity, making them indispensable for modern technologies. In contrast, natural plasma, despite its scientific and observational value, is inherently chaotic, variable, and limited in its practical use.
Uniform plasma enables consistent results in processes like semiconductor manufacturing, materials science, and aerospace engineering. By ensuring uniform distribution of energy and chemical reactions, artificial plasma systems offer:
As artificial plasma systems evolve, achieving and maintaining uniformity will remain critical to optimizing outcomes and expanding their applications across industries.
The uniformity of plasma is closely tied to its temperature characteristics, dividing it into three broad categories: hot, warm, and cold plasma.
Hot Plasma
Hot plasma, found in high-energy applications such as fusion reactors and arc welding, features extremely high temperatures where electrons and ions exist in thermal equilibrium. These plasmas are typically non-uniform because they form under chaotic and uncontrolled circumstances. Despite their instability, hot plasma is invaluable; however, their lack of control and uniformity prevents their practical use in precision-driven technologies.
Warm Plasma
Warm plasma exists in intermediate states, often in artificial systems that strive to bridge the gap between hot and cold plasmas for industrial applications. Although more controlled and uniform compared to hot plasma, warm plasma still presents challenges in maintaining stability and uniformity, which limits its applications and makes them less diverse than those of cold plasma.
Cold Plasma
Characterized by non-equilibrium conditions where electron temperature is significantly higher than ion or gas temperature, cold plasma is the most extensively used in industrial applications. Due to its lower overall temperature, cold plasma can be generated, manipulated, and engineered to achieve the high degree of precision required for uniformity. Applications of cold plasma are widespread, ranging from microelectronics (etching and deposition) to food sterilization, cutting-edge medicine (such as wound healing and cancer treatment), and aerospace surface treatments.
While hot and warm plasmas can be studied for energy generation (nuclear fusion), it is cold plasma systems that push the boundaries of modern industrial and scientific advancements. Their ability to achieve exceptional uniformity makes them essential for next-generation technologies.
-----
Plasma uniformity is integral to the success of cold plasma applications, making it a cornerstone of modern industries. As research continues to optimize uniformity and scalability, the potential for cold plasma to revolutionize fields such as manufacturing, medicine, and energy remains enormous. In contrast, hot plasma, while crucial for energy research and industrial applications, lacks the stability and control to support precision-driven applications. By bridging the gap between scientific theory and industrial engineering, plasma uniformity will shape future innovations and enable the development of transformative technologies.
Plasma stability refers to the ability of a plasma system to maintain a stable state or predictable behavior despite internal or external disturbances. In natural plasmas, such as those in stars or planetary magnetospheres, stability arises from inherent physical phenomena, including gravitational forces, thermal dynamics, and magnetic fi
Plasma stability refers to the ability of a plasma system to maintain a stable state or predictable behavior despite internal or external disturbances. In natural plasmas, such as those in stars or planetary magnetospheres, stability arises from inherent physical phenomena, including gravitational forces, thermal dynamics, and magnetic fields. In contrast, artificial plasmas, created and controlled by humans, rely on engineered stability to meet the precise demands of specific applications. This includes deliberately balancing key forces, such as electric and magnetic fields, to ensure efficient, safe, and reproducible performance.
Stability is a critical factor across all plasma types, including cold, warm, and hot. However, the degree of stability and the methods used to maintain it differ significantly based on the plasma's thermal and ionization characteristics.
Cold Plasma
Cold plasmas are characterized by low energy and partial ionization, offering unique stability advantages due to their lower temperature and ease of manipulation via electric and magnetic fields. Their relatively low thermal pressure makes them less prone to turbulence or destabilizing temperature fluctuations, allowing them to be stabilized more effectively using feedback controls and externally regulated parameters. This makes cold plasmas ideal for applications where precision and predictability are crucial, such as material processing, sterilization, and medical therapies.
The reduced complexity of cold plasma environments allows for predictable behavior, making them ideal for precision-driven applications. In industries such as material processing, sterilization, and medical therapies, where consistent outcomes are critical, cold plasmas deliver unmatched reliability. For example, in plasma etching or medical sterilization, electric fields can be precisely controlled to regulate particle motion and dynamics, ensuring uniform results.
Warm Plasma
Warm plasmas occupy an intermediate state, with higher temperatures and a greater degree of ionization than cold plasmas. Their stability results from balancing thermal pressure and external magnetic fields. However, this balance is inherently less stable compared to cold plasmas, as warm plasmas experience greater susceptibility to thermal fluctuations and pressure gradients. To maintain stability, warm plasmas often require significant external intervention, which can complicate their control mechanisms and limit their consistency.
Hot Plasma
Hot plasmas, such as those found in fusion reactors, operate at extremely high temperatures, where particles are fully ionized. In these environments, stability is an ongoing challenge due to the presence of high temperatures, turbulence, and severe pressure gradients. Advanced confinement techniques, such as magnetic confinement in tokamaks or inertial confinement in laser-driven systems, are essential for managing the instabilities inherent to hot plasmas. Despite these efforts, achieving long-term stability remains a significant challenge to the practical application of nuclear fusion energy.
Key Forces in Plasma Stability
The interplay of several fundamental forces governs stability across all plasma types. However, the specific role of these forces varies significantly between cold, warm, and hot plasmas:
In cold plasma systems, external controls, including advanced algorithms and feedback mechanisms, are used to manage these forces proactively, providing a level of stability that is largely unmatched by high-temperature plasmas.
-----
Plasma stability, especially in cold plasma systems, underpins the reliability and scalability of various technologies. The ability to precisely manipulate conditions in cold plasma enables transformative applications in medicine, industry, and environmental protection. Unlike hot plasmas, where stability management is a constant struggle due to high temperatures and pressures, and warm plasmas, which must contend with natural variability, cold plasmas offer a more manageable and versatile platform.
Research into improving stability across all plasma types remains critical for expanding their capabilities. However, the unique balance of low thermal pressure and strong external control in cold plasma positions it as an indispensable tool in next-generation innovations. By continuing to refine control methodologies and address emerging instability risks, cold plasma systems will expand their role in solving complex problems across a diverse range of fields.
Density is a key parameter in plasma physics, directly tied to the concentration of particles—such as electrons—within a given volume. This characteristic governs plasma behavior, influencing its electrical conductivity, chemical reactivity, stability, and interactions with electromagnetic fields. Plasma density can be categorized into tw
Density is a key parameter in plasma physics, directly tied to the concentration of particles—such as electrons—within a given volume. This characteristic governs plasma behavior, influencing its electrical conductivity, chemical reactivity, stability, and interactions with electromagnetic fields. Plasma density can be categorized into two types: particle density, which encompasses all charged and neutral species, such as atoms, ions, and electrons, and electron density, which specifically measures the abundance of free electrons. Electron density plays a central role in determining the fundamental dynamics of plasma and serves as a critical metric for both theoretical research and practical applications. It is typically expressed in terms of particles per cubic meter (m³) or free electrons per cubic centimeter (cm³), depending on the context and scale.
Electron density is crucial in cold plasma, where only a fraction of the atoms or molecules are ionized, resulting in distinct behaviors compared to hot and warm plasmas. Electron density is simply how many free electrons exist per cubic centimeter, whereas ionization refers to the proportion of atoms and molecules that have been ionized. These two are related, but they don't always grow linearly. A plasma can have a lower electron density but still be more ionized if the total number of particles (e.g., atoms or molecules) in the plasma is initially lower. Understanding and controlling electron density in cold plasma is crucial for optimizing its application across various fields, including medical treatments, materials processing, and environmental remediation.
Plasmas can be broadly classified as cold, warm, or hot, depending on their thermal energy and corresponding electron density. These distinctions are directly tied to the plasma's temperature, level of ionization, and energy environment:
Cold Plasma
Cold plasmas are weakly ionized, meaning only a small fraction of gas molecules or atoms are ionized. However, due to the abundance of neutral particles in the environment, even weak ionization can generate a substantial number of free electrons. This leads to electron densities typically ranging from 10^11 to 10^13 electrons per cubic centimeter (cm³), equivalent to 10^17 to 10^19 electrons per cubic meter (m³). The majority of neutral particles remain at near-room temperature, while electrons possess higher energy levels, creating non-equilibrium conditions.
These unique ionization properties allow cold plasma to efficiently generate reactive species (e.g., free radicals, ions) while avoiding significant thermal damage to the surrounding environment. Such properties enable cold plasma to safely interact with various surfaces, living tissues, and liquids, making them widely useful in medicine (e.g., wound healing, sterilization), agriculture, and environmental remediation.
Warm Plasma
Warm plasmas are partially ionized and occupy an intermediate state between cold and hot plasmas. They arise in conditions with higher levels of ionization and thermal energy than cold plasmas but remain below the extremes of hot plasmas. Warm plasmas exhibit moderate electron densities (ranging from 10^9 to 10^13 electrons/cm³), equivalent to 10^15 to 10^19 electrons/m³, depending on the specific conditions. Although warm plasmas are typically found in environments where the degree of ionization is higher than cold plasma, the total number of particles in warm plasma is often less than in cold plasma (due to different conditions like temperature and pressure), so the overall electron density can be lower despite the plasma being more ionized.
Warm plasmas play a crucial role in applications that involve electromagnetic wave propagation, space weather studies, and satellite communications. Their moderately ionized nature and electron density make them highly significant in technological applications.
Hot Plasma
In contrast, hot or fully ionized plasma, meaning that nearly all atoms or molecules are stripped of their electrons. They exhibit extremely high electron densities, often in the range of 10^11 to 10^14 electrons/cm³ or 10^17-10^20 electrons /m³ or higher, depending on the environment. In such plasmas, nearly all atoms are ionized, resulting in high electron densities, strong electrical conductivity, and intense thermal and magnetic processes.
Hot plasmas rely on high-energy environments, where frequent collisions among particles ensure a uniform distribution of energy. This property is critical in sustaining nuclear fusion reactions, as high electron densities support repeated collisions necessary to achieve the required temperatures and pressures. These conditions are also essential for simulating extreme astrophysical environments.
The differences among hot, warm, and cold plasmas illustrate how electron density fundamentally determines plasma behavior. While hot plasmas rely on high densities to sustain energy-intensive processes, cold plasmas leverage lower densities to achieve greater selectivity and precision in reactive processes.
Measurement and Importance of Electron Density
Electron density is a crucial parameter in determining plasma behavior and optimizing its utilization. Techniques like Laser-Induced Fluorescence (LIF), microwave reflectometry, and Thomson scattering enable accurate measurements by observing plasma interactions with electromagnetic waves. These measurements are essential for understanding and improving plasma applications across various domains, including medical treatments, materials processing, and nuclear fusion.
Chemical composition refers to the types and concentrations reactive species—such as ions, radicals, and neutral species—within the plasma environment. In cold plasma, the specific chemical composition is crucial in determining its reactivity, selectivity in chemical reactions, and physical properties. Understanding and controlling this c
Chemical composition refers to the types and concentrations reactive species—such as ions, radicals, and neutral species—within the plasma environment. In cold plasma, the specific chemical composition is crucial in determining its reactivity, selectivity in chemical reactions, and physical properties. Understanding and controlling this composition is essential for engineering plasma in various applications, including surface modification, material synthesis, sterilization, and semiconductor manufacturing. Cold plasma is distinct in that it operates at low temperatures (around room temperature), allowing for greater flexibility when interacting with temperature-sensitive materials compared to warm or hot plasmas.
Plasma is categorized based on temperature differences into three types: cold, warm, and hot plasma, which influence its chemical behavior and composition.
Cold Plasma
Cold plasma is partially ionized, meaning it contains a higher proportion of neutral species alongside charged particles, including ions and electrons. Its low temperature ensures that electrons maintain high energy levels while the bulk gas remains close to ambient temperature. This leads to the production of reactive species, such as ions, radicals, and excited atoms, while minimally affecting the thermal state of surrounding materials or gases.
The chemical composition of cold plasma is heavily dependent on the gas used (e.g., oxygen, nitrogen, or argon) and the external conditions, such as energy input and pressure. For example, oxygen plasma generates reactive oxygen species (ROS), such as ozone (O₃), atomic oxygen, and singlet oxygen, which are valuable for sterilization and etching processes. These reactive species make cold plasma an efficient tool for applications that require high reactivity without thermal degradation of materials.
Warm Plasma
Warm plasma, characterized by intermediate temperatures, exhibits increased ionization and greater thermal energy compared to cold plasma. While reactive species such as radicals and ions are still present, the higher temperature of warm plasma may accelerate secondary chemical reactions, sometimes reducing selectivity by allowing multiple pathways to occur simultaneously. This can be beneficial for processes like rapid material deposition, but may hinder delicate or precise applications where specific reactions are required.
Hot Plasma
Hot plasma is fully ionized and operates at extremely high temperatures, typically several thousand kelvins. Due to the nearly complete ionization of atoms, their chemical composition is dominated by highly energetic charged particles. The intense heat drives high levels of reactivity, enabling the breakdown of even the most stable chemical bonds. However, the high temperature significantly limits its practical applications to heat-tolerant systems, such as nuclear fusion, astrophysical studies, or industrial processes requiring extreme energy transfer.
Compared to hot and warm plasmas, the chemical composition of cold plasma provides greater versatility. Its low temperature reduces constraints on the materials it can interact with, while its partially ionized nature delivers sufficient reactivity without introducing excessive energy. This allows cold plasma to strike a balance between chemical precision and gentleness, making it ideal for surfaces with specific structural or thermal limitations.
Critical Aspects of Chemical Composition
The distinct chemical composition of cold plasma allows it to exhibit unique behavior and properties. Critical aspects of this composition include:
-----
The chemical composition of cold plasma is important to its effectiveness, enabling selective and precise chemical reactions without introducing the thermal constraints associated with warm or hot plasma. This unique property stems from its partially ionized state and dynamic mixture of neutral and charged species, which deliver high reactivity at low temperatures. By harnessing and controlling this composition, cold plasma continues to drive innovation in sterilization, material modification, and microelectronics manufacturing. As our understanding and control of its chemical composition evolve, cold plasma will be an essential part of cutting-edge technologies, offering highly efficient, application-specific solutions in both industrial and scientific fields.
Plasma is characterized by its exceptional chemical reactivity, which stems from the presence of charged particles such as electrons and ions, as well as reactive species, and neutral atoms and molecules. These components create a highly energetic environment where chemical reactions, such as dissociation, ionization, and radical formatio
Plasma is characterized by its exceptional chemical reactivity, which stems from the presence of charged particles such as electrons and ions, as well as reactive species, and neutral atoms and molecules. These components create a highly energetic environment where chemical reactions, such as dissociation, ionization, and radical formation, can occur. Importantly, these reactions are often unattainable through conventional chemical methods, making plasma a valuable tool for numerous scientific and industrial applications.
While all plasmas exhibit high reactivity due to their charged and excited particles, the extent and nature of their reactivity vary depending on their thermal state—specifically, whether they are cold, warm, or hot plasmas. Understanding these differences provides further insight into their respective advantages, particularly in applications that require precise chemical control.
Cold Plasma
Cold plasma is unique in that its electrons are highly energetic, while the bulk gas remains near room temperature. This state creates an environment where chemical reactivity is decoupled from thermal energy. Cold plasma drives reactions through the production of highly reactive species, causing minimal heat damage. This distinctive property makes cold plasma highly advantageous in applications requiring precision and the preservation of sensitive materials.
Warm Plasma
Warm plasma occupies an intermediate state, with moderate particle energies and temperatures. This type of plasma is used in applications such as plasma arcs for welding and cutting, where significant energy is required. Still, precision is not as critical as in other applications.
Hot Plasma
Hot plasma, commonly found in fusion reactors, is in a state of thermal equilibrium, where electrons, ions, and neutral particles possess extremely high temperatures, on the order of millions of degrees. Its high particle energy creates extreme reactivity, enabling nuclear fusion and processes that require immense energy input. However, this reactivity is less suited for delicate or targeted applications due to the destructive nature of high thermal energy.
-----
Cold plasma's exceptional reactivity, driven by its production of energetic electrons, ions, and radicals at low temperatures, positions it as a transformative tool across numerous industries. Unlike hot and warm plasmas—which rely on high thermal energy for reactivity—cold plasma achieves chemical transformations with precision and minimal thermal impact, making it indispensable for sensitive and high-precision applications. From microelectronics fabrication to sterilization, the ability of cold plasma to selectively drive reactions without damaging substrates defines its significance. The comprehensive understanding and manipulation of cold plasma reactivity will continue to be essential in the development of advanced technologies and innovative processes. Its reactivity is not just a characteristic—it is the foundation of its broad applicability and forward-looking potential.
Plasma exhibits remarkable energy transfer properties. The efficiency of energy transfer within plasma environments refers to how effectively energy is distributed, utilized, and retained for intended applications. High energy transfer efficiency reduces energy losses, enhances performance, and optimizes sustainability, making it a critic
Plasma exhibits remarkable energy transfer properties. The efficiency of energy transfer within plasma environments refers to how effectively energy is distributed, utilized, and retained for intended applications. High energy transfer efficiency reduces energy losses, enhances performance, and optimizes sustainability, making it a critical focus in plasma research and technological advancements. Cold plasma, in particular, holds significant potential due to its unique properties and application versatility, offering a transformative approach to improving energy transfer efficiency across numerous fields.
Plasma is classified into three types based on temperature: cold, warm, and hot plasma. These temperature ranges influence energy transfer efficiency and determine practical applications.
Cold Plasma
Cold plasma operates at a much lower temperature compared to warm or hot plasma, with electron temperatures substantially higher than the neutral gas temperature. This disparity allows for selective interactions, where energy is efficiently directed toward ionization and the formation of reactive species, rather than heating the bulk material. Cold plasma excels in energy transfer efficiency for applications requiring precision, such as medical treatments, surface modification, and atmospheric pressure plasma systems. It is crucial in scenarios where minimizing excess energy dissipation is critical for achieving desired outcomes.
Warm Plasma
Warm plasma features intermediate temperatures, where both electrons and ions carry moderate thermal energy. While warm plasma systems balance energy transfer and thermal effects, they tend to exhibit reduced efficiency compared to cold plasma due to non-selective energy dissipation and higher thermal losses. Warm plasma systems are used in applications such as plasma processing and certain propulsion technologies, where moderate energy levels are appropriate for specific reactions.
Hot Plasma
Hot plasma operates at extremely high temperatures, typically found in fusion reactors and arc welding. This type of plasma demands significant energy input and often struggles with energy transfer efficiency due to challenges such as particle collisions, radiation losses, and maintaining plasma confinement. While necessary for high-energy applications like nuclear fusion, hot plasma tends to incur larger energy losses, making it less efficient compared to cold plasma in applications where precise energy usage is required.
Mechanisms Governing Energy Transfer
Efficiency
Cold plasma exhibits high energy transfer efficiency due to several key mechanisms:
-----
Cold plasma demonstrates unparalleled energy transfer efficiency due to its ability to direct energy toward specific reactions while minimizing waste selectively. Compared to warm and hot plasma, cold plasma's lower temperatures and targeted mechanisms make it indispensable in applications requiring precise energy utilization and minimal dissipation. From medical innovations to space propulsion, cold plasma technology drives the development of sustainable, efficient, and transformative solutions. Its development and optimization stand at the forefront of advancing energy-efficient plasma systems, underscoring its importance in modern science and industry.
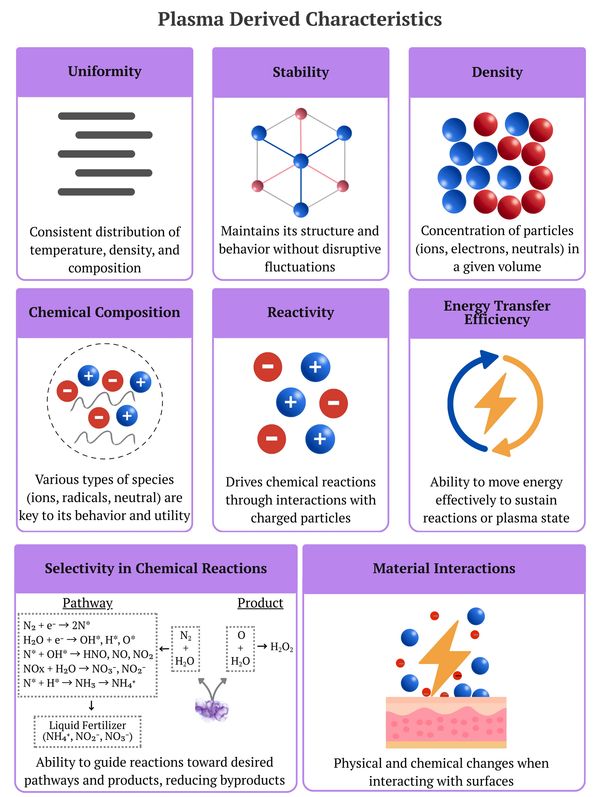
Selectivity in chemical reactions refers to the ability to control reaction pathways to produce specific desired products while minimizing the formation of undesired by-products. This concept is crucial for optimizing reaction efficiency, reducing waste, and improving overall process sustainability. Cold plasma, which operates at relative
Selectivity in chemical reactions refers to the ability to control reaction pathways to produce specific desired products while minimizing the formation of undesired by-products. This concept is crucial for optimizing reaction efficiency, reducing waste, and improving overall process sustainability. Cold plasma, which operates at relatively low temperatures compared to hot or warm plasma, offers unique advantages in achieving high selectivity in chemical reactions due to its ability to precisely manipulate reaction conditions.
Plasma is a highly energetic mixture of ions, electrons, radical species, and neutral particles. Different types of plasma—cold, warm, and hot—vary in temperature and energy distribution, with these differences significantly influencing their impacts on chemical selectivity.
Cold Plasma
Cold plasma operates at low temperatures where the gas remains near room temperature while electrons are highly energized. This temperature disparity allows for the selective activation of specific chemical bonds or intermediates without excessive thermal excitation. This distinct feature of cold plasma minimizes unwanted side reactions, creating a highly controlled environment for precise chemical transformations.
Warm Plasma
Warm plasma refers to systems with intermediate energy levels, where the temperature differences between electrons and heavy species (ions and neutrals) are less pronounced than in cold plasma. Warm plasma can support specific reactions but often struggles to achieve the high degree of selectivity needed in chemical processes due to significant thermal activation and broader species interactions.
Hot Plasma
Typical of thermonuclear processes, hot plasma is characterized by extremely high temperatures, often exceeding thousands or millions of degrees. These conditions generate intense energy but lack the refined control needed for selective chemical reactions. High temperatures indiscriminately activate various species and reaction pathways, leading to a more chaotic reaction environment with reduced specificity.
Key Mechanisms Enabling Selectivity
Cold plasma's ability to achieve superior selectivity stems from its capacity to precisely control critical reaction parameters, including energy distribution, reactive species concentrations, and reaction dynamics:
-----
Cold plasma represents a powerful platform for achieving precise chemical selectivity, offering unparalleled control over reaction pathways compared to warm or hot plasma systems. Its ability to selectively activate specific bonds, stabilize reactive intermediates, and precisely control reaction conditions makes it indispensable for applications in chemical synthesis, material engineering, surface functionalization, and environmental remediation. The low-temperature operation of cold plasma minimizes side reactions and enhances efficiency, addressing many challenges faced in traditional chemistry.
As research in plasma science advances, harnessing the unique properties of cold plasma will be critical in driving sustainable and efficient chemical processes, making it an essential tool for future innovation in industrial and scientific fields.
Material interactions in plasma environments involve the physical and chemical exchanges between reactive plasma species and the surface of materials. These interactions result in surface transformations, such as etching, chemical functionalization, and changes in microstructural or chemical composition. Understanding these mechanisms is
Material interactions in plasma environments involve the physical and chemical exchanges between reactive plasma species and the surface of materials. These interactions result in surface transformations, such as etching, chemical functionalization, and changes in microstructural or chemical composition. Understanding these mechanisms is key to improving plasma-based processes widely used in industries such as electronics, materials science, and biotechnology.
Plasma exists in various forms: hot, warm, and cold. These types differ primarily in terms of temperature and energy distribution, which in turn influence their impact on material surfaces. Hot plasma, found in environments like fusion reactors or welding, contains high-energy ions and electrons that can destroy or vaporize most materials, making it unsuitable for precise surface treatments. Warm plasma, characterized by moderate energy levels, is more controlled but still limited in its ability to treat delicate or heat-sensitive surfaces. In contrast, cold plasma, where the ionized gas remains at near-room temperature, provides a highly selective and energy-efficient platform for material interactions. Cold plasma is particularly advantageous for industries that require precise surface modifications without damaging underlying materials, such as those involved in the manufacture of sensitive electronic and biomedical devices.
Mechanisms of Plasma-Material Interactions
Understanding the fundamental mechanisms by which plasma interacts with materials is critical for engineering its applications. These processes involve:
-----
Material interactions within plasma environments are foundational to many modern technologies, particularly in fields seeking precise and efficient surface modifications. While hot and warm plasmas have specific uses, they often pose limitations due to thermal damage or lack of fine control. Cold plasma, with its reactive yet low-energy nature, emerges as the most versatile tool for industrial and biomedical applications requiring precision.
The unique ability of cold plasma to balance reactivity and thermal control makes it indispensable for applications like microfabrication, thin-film deposition, and biomedical device engineering. Enhancing our understanding of plasma-material interactions, particularly in the context of cold plasma, will continue to drive innovation, ensuring more efficient, functional, and sustainable solutions across diverse industries.
Plasma is well known for its characteristic glow—a visible emission of light that distinguishes it from solids, liquids, and gases. This glow is not a universal feature of every plasma but is particularly prominent in many artificial plasmas. It arises from specific energy interactions and is a powerful diagnostic and functional hallmark
Plasma is well known for its characteristic glow—a visible emission of light that distinguishes it from solids, liquids, and gases. This glow is not a universal feature of every plasma but is particularly prominent in many artificial plasmas. It arises from specific energy interactions and is a powerful diagnostic and functional hallmark of plasma systems.
Plasma glows through a fundamental process of energy excitation and light emission. When electrons within the plasma are energized—commonly by an external energy source (e.g., an electric or electromagnetic field)—they transition to higher energy states. As these electrons return to their more stable, lower-energy states, they release photons of light. The precise color of this emitted light depends on the type of gas present in the plasma and the nature of the energy transitions. For example, neon plasma emits a distinct reddish-orange glow, while argon and krypton plasmas produce blue and violet hues, respectively. The unique glow of a plasma, therefore, acts as a spectral fingerprint that can reveal its chemical composition and energy profile.
The phenomenon of plasma glow varies significantly depending on the temperature and nature of the plasma, which can be broadly categorized as cold, warm, or hot.
Cold Plasma
Unlike hot plasmas, cold plasmas operate at much lower overall temperatures while maintaining highly energized electrons that drive ionization and produce a glow. This makes their light emissions distinct and functional, as cold plasmas are often used in controlled environments, such as in neon lights, plasma displays, and numerous industrial applications. Unlike high-temperature plasma, the glow of cold plasma is deliberately engineered for specific purposes, whether for diagnostics, processing, or aesthetics.
Warm Plasma
Warm plasma, such as atmospheric discharges, exists between hot and cold types. While their glow is generally less intense than that of hot plasmas, it still reflects the interplay of ionization and energy transitions in semi-controlled environments. The glows of auroras, for instance, occur due to collisions between charged particles from solar winds and Earth's atmospheric gases, creating vivid, unpredictable displays.
Hot Plasma
Found in environments such as fusion reactors or welding, hot plasmas emit light primarily due to extremely high temperatures, which fully ionize gas molecules. In these cases, the intense glow results from a tremendous release of energy, with chaotic and highly variable light emissions. For example, the sun's plasma emits across a broad spectrum, creating continuous, dazzling brightness. However, due to such extreme energies, the glow is often a byproduct of the overwhelming thermal processes, not a focus of diagnostic control.
Plasma Glow: A Controlled Feature of Cold Plasma
In cold plasmas, the glow is far more than a visual phenomenon. It serves as a critical diagnostic and functional tool in scientific and technological applications. For example, the intensity, color, and uniformity of the plasma glow are regularly analyzed in real-time to monitor properties such as electron density, ionization efficiency, and gas purity. This is especially true in industries such as semiconductor manufacturing and plasma-based medical technologies, where precision control over plasma properties is paramount. For example, in semiconductor manufacturing, the plasma glow provides real-time feedback on etching or deposition processes, ensuring accurate fabrication of microstructures. In plasma medicine, the glow of cold plasma helps optimize its therapeutic effects in skin treatments or sterilization by indicating effective ionization levels without thermal damage to surrounding tissues.
-----
The glowing property of plasma encapsulates its dual scientific and practical significance, especially in cold plasma systems. While natural plasmas, such as stars and auroras, showcase plasma's inherent ability to emit captivating light, it is in cold plasma that the glow becomes a highly controlled, diagnostic, and functional feature. This unique glow serves as a testament to the dynamic interactions of charged particles and as a tool for innovation, enabling precise monitoring, advanced manufacturing, and groundbreaking medical applications.
Understanding the vibrational and rotational states of molecular species is essential for advancing plasma-based technologies, particularly within the realm of cold plasma. These states directly influence energy dynamics, chemical reactivity, and emission characteristics in plasma, making their study critical for optimizing artificial pla
Understanding the vibrational and rotational states of molecular species is essential for advancing plasma-based technologies, particularly within the realm of cold plasma. These states directly influence energy dynamics, chemical reactivity, and emission characteristics in plasma, making their study critical for optimizing artificial plasma applications in medicine, materials processing, and spectroscopy.
Both vibrational and rotational transitions are integral to plasma processes, with their interplay determining how plasma interacts with matter and influences system stability and efficiency.
Vibrational and rotational transitions vary significantly depending on the temperature and nature of the plasma, which can be broadly categorized as cold, warm, or hot.
Cold Plasma
Cold plasma, characterized by its low gas temperature (typically near ambient) and high electron energy, is particularly sensitive to vibrational and rotational states. Unlike hot and warm plasmas, where thermal energy dominates and molecules often dissociate or ionize completely, cold plasma enables precise control of molecular excitation without excessive thermal degradation.
Cold plasma focuses energy into its electrons rather than raising the overall gas temperature. This decoupling enables selective excitation of vibrational and rotational states without significant atomization of molecules. This is crucial for applications that require controlled interaction with matter, such as plasma medicine, environmental monitoring, and precision materials processing.
In cold plasma, higher vibrational and rotational states are often selectively excited, enabling targeted reactions at lower average temperatures. This precision facilitates efficient energy transfer and chemical modifications, particularly in non-equilibrium conditions where chemical reactions are driven by specific molecular alignments rather than bulk heating.
Warm Plasma
Warm plasma, often found in industrial applications like electric arcs or welding, operates at intermediate temperatures. While molecules may still exhibit vibrational and rotational motions, these states frequently transition to dissociated or partially ionized states due to collisional heating. This makes precise control of molecular states challenging, as energy transfer is less localized compared to cold plasma.
Hot Plasma
Hot plasma exists at extremely high temperatures (e.g., fusion reactors), where molecular bonds break down into atomic or fully ionized species. Vibrational and rotational states are largely irrelevant in such environments, as molecules rarely exist long enough to sustain these intermediate states. Energy is distributed uniformly among all species, making it impossible to control molecular transitions.
-----
The vibrational and rotational states of molecular species are critical for understanding the unique properties and applications of cold plasma. Unlike hot or warm plasma, where thermal energy dominates, cold plasma allows precise manipulation of these molecular states. This selective excitation enables efficient energy transfer and controlled reactivity, powering groundbreaking applications in medicine, materials science, and environmental monitoring. By optimizing the interactions between vibrational and rotational states, cold plasma continues to unlock new possibilities for precision, innovation, and sustainable technological advancements.
Copyright © 2025 What is Cold Plasma? - All Rights Reserved.